Deze pagina is momenteel niet beschikbaar in uw taal. U kunt met behulp van Google Translate een automatische vertaling bekijken. Wij zijn niet verantwoordelijk voor deze dienstverlening en het vertaalresultaat is niet door ons gecontroleerd.
Heeft u meer hulp nodig, neemt u dan contact met ons op.
A healthy look at plants – Raman imaging of plant chemistry
Most of us take the existence of plants for granted. After all, they have always been here, and always will be but is that really true?
Plants - like all species on our planet - evolve, adapting to changing climates and habitats, and increasing urbanization. In the past, plants had time to gradually adjust to external factors, but can they keep up with the current pace of human-driven changes? Plants are vital to us as they provide 98% of the oxygen we breathe and 80% of our food. They significantly impact our environment and economy. [1] For these important reasons, 2020 has been declared by the United Nations as the International Year of Plant Health (IYPH). [2]
Many factors can actively influence plant growth, their health, and resistance to disease. It is vital that we monitor plants and detect changes taking place, ideally before the visible signs of them occur. We therefore need suitable analytical techniques.
Raman spectroscopy is ideal for this. It gives highly specific chemical information, is non-destructive, and can analyse samples in water. This enables researchers to measure biological processes ex-vivo and in-vivo.
Here I illustrate some research on plants, made using Renishaw's inVia confocal Raman microscope. It is a research grade instrument, well suited to analysing plant tissue. Its advanced hardware and software enables it to image botanical samples with a high spatial resolution and specificity.
From kernel to wood
Grains
Cereal grains are one of the main food sources of our diet. Their structure and chemical composition directly influence their industrial processability, and how they are broken down in human and animal digestion systems.
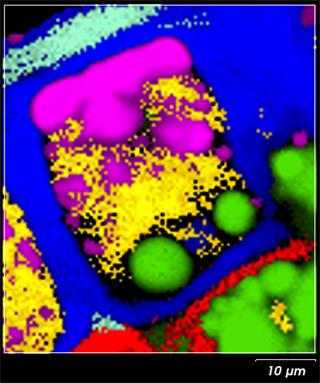
Raman imaging enables us to reveal the spatial distribution of components in a cross section of a kernel. Figure 1 shows the Raman image of a wheat grain cross-section. Specific sections of the kernel structure can be easily distinguished, including aleurone layer cell (centre), starchy endosperm (left bottom corner), aleurone-pericarp border (right top corner). [3] By interpreting distributions such as these we can understand the effect of external factors on plants and their seeds. That supports a development of more robust grain varieties.
Metabolites
If we can track the effect of environmental, nutritional and mechanical stresses on a plant's tissues we can reveal their impact on the plant's health and development. [4]
Different types of metabolites can form in a plant's tissues and organs (roots, wood, stems, bark, leaves, seeds, etc.). If we can monitor and understand metabolite production, we gain insights into how plants adapt to environmental changes, and how their growth will be affected by climate change.
Calcium oxalate is a key metabolite. It regulates calcium levels in tissues, protects against herbivores, detoxifies heavy metals, strengthens tissues, and helps in the gathering of light for photosynthesis. Its amount, crystal shape, size and function are defined by a combination of genetic and environmental factors. [5]
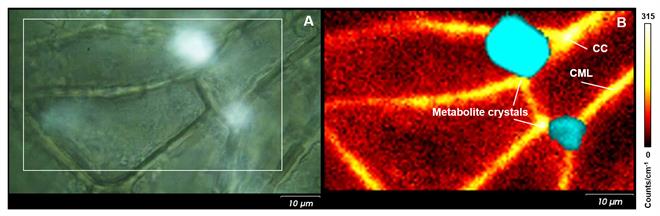
You can easily study the spatial distribution of calcium oxalate crystals in plant tissues and organs with Raman spectroscopy. Figure 2 shows an optical image of an English oak leaf, and the corresponding Raman image. The Raman image shows the lignin content, with its highest content at the compound middle lamella (CML) and cell corners (CC). The large calcium oxalate metabolite crystals are present in the corners of the leaf cells (Fig. 2B; cyan). We can also use Raman spectroscopy because of its high chemical specificity to study the hydration state of calcium oxalate.
Wood
Trees are vital to us. They produce oxygen for us to breathe and give space for wildlife to live. They are also crucial to our economy, being used in the production of paper, furniture, and buildings.
The more we understand the complex structure of wood tissues, the more we can predict their mechanical durability and resistance to decomposition and biological attack.
Wood consists primarily of cellulose, hemicelluloses, and lignin. We can use the high specificity of Raman spectroscopy to study the structure and organization of wood cell walls. Their resistance to decomposition and biological attack strongly depends on the presence of the secondary metabolites called extractives.
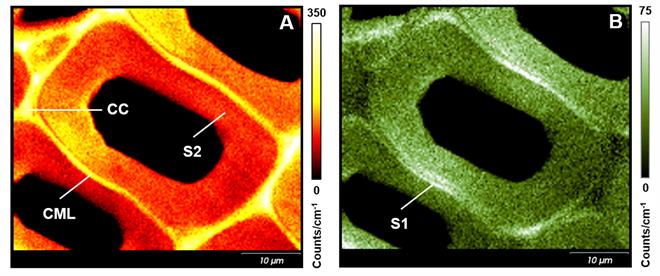
Image (A) of Figure 3 shows the distribution of lignin in a cross-section of Scots Pine. Lignin is concentrated at the compound middle lamella (CML; middle lamella + adjacent primary walls) and cell corners (CC), with a higher concentration than in the sublayer of the secondary cell wall (S2). Image (B) was generated using a polarised laser to reveal the variation in cellulose orientations. Two small layers next to the CML, with a high intensity of the cellulose band, appear to reveal a sublayer of the secondary cell wall (S1).
Bright Future
The pace of the observed changes in the environment accelerates each year. This also applies to plants. The successful approach to the new reality requires faster and simpler analytical methodologies. In this context, Raman imaging is playing an increasingly important role in the study of plant tissues and organs. This is because it is versatile, providing images, chemical and spatial analysis, and information on molecular orientation.
The research results generated using Raman spectroscopy can be a key element in the new approach to the studies on plant health. It can be a missing puzzle piece, in which inVia confocal Raman microscope will play a crucial role.
About the author
Anna Lewandowska, Application Scientist

Anna received her PhD in Physical Chemistry from Adam Mickiewicz University in Poland. She's successfully developed research in both fields of Materials Science and Analytical Methods, particularly focusing on a preparation of materials and development of characterisation methodologies.
With over 16 years of experience in Raman spectroscopy she has investigated heterogeneous catalysts, solid materials, fibres, composites, cellulose and plant-based tissue. She has extensive knowledge in the combined in situ spectroscopy with GC/MS measurements for real-time chemical processes monitoring and has also used in situ methodology for micro-mechanical tests of fibres and composites in engineering.
References
- https://www.yearofplanthealth.co.uk/why-plants-are-important
- http://www.fao.org/plant-health-2020/home/en/
- A.-S. Jskelinen, U. Holopainen-Mantila, T. Tamminen, T. Vuorinen, J. Cereal Sci. 57 (2013) 543-550
- H.J. Butler, M.R. McAinsh, S. Adams, F.L. Martin, Anal. Methods, 7 (2015) 4059-4070
- M.N. Islam, M. Kawasaki, Plant Prod. Sci. 17 (2014) 13-19