Fluorescence lifetime imaging microscopy (FLIM)
Correlated FLIM-Raman analysis on Renishaw's inVia™ confocal Raman microscope.
Renishaw is pleased to offer an integrated solution for fluorescence lifetime imaging microscopy (FLIM) on an inVia™ confocal Raman microscope. This enables fluorescence lifetime images to be seamlessly measured, analysed, and overlaid with Raman data.
What is FLIM?
After light interacts with a fluorophore, the fluorescence lifetime is measured. The fluorescence lifetime is the characteristic time a fluorophore remains in an excited electronic state before returning to the ground state by emitting a photon. Imaging based on the measurement of this lifetime is called fluorescence lifetime imaging microscopy (FLIM).
The lifetime of a fluorescence decay is sensitive to the molecular environment and resulting changes in molecular conformation. The FLIM technique can be used to study a range of properties:
- pH/ion/oxygen concentration
- molecular binding
- proximity of energy acceptors
FLIM has the following advantages over intensity-based measurements: FLIM is independent of fluorophore concentration; and FLIM is an internally self-referenced measurement, so it does not require calibration to account for instrument configuration.
FLIM is particularly applicable to research in cell biology and can be used for environmental sensing, monitoring molecular interactions, cancer diagnosis, live cell imaging and fluorophore identification.
Why correlate FLIM with Raman?
Correlative FLIM and Raman microscopy are complementary methods for label-free imaging of biological cells and tissue sections. FLIM images provide insight into molecular interactions and can be obtained at least 10x faster than Raman images. This makes it an ideal technique to identify regions of interest for subsequent chemical and structural characterisation by Raman imaging.
Our solution
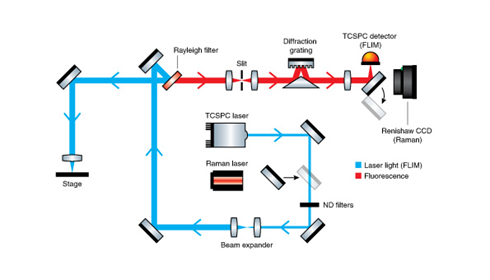
Renishaw has partnered with Becker & Hickl to produce a completely integrated time-correlated single photon counting (TCSPC) FLIM Raman microscope. TCSPC FLIM measures the time between incident light on the sample and the emitted light's arrival at the detector. A 405 nm pulsed laser (<40 ps pulse width) is used to stimulate photon emission/fluorescence. (Other laser wavelengths are available on request.) The emitted fluorescence light is detected using a single-photon avalanche diode (SPAD) detector (timing resolution of <40 ps).
The FLIM image is then created by conducting TCSPC at multiple points on the sample. Our FLIM Raman system combines Raman mapping and FLIM on the same Renishaw MS30 stage. This enables easy pixey-by-pixel correlation of data using the same measurement co-ordinates.
For FLIM data analysis, comprehensive TCSPC FLIM software from Becker & Hickl is included with multi exponential, incomplete and shifted component analysis models. The software also includes proprietary phasor analysis to distinguish lifetime populations. Fluorescence lifetime and Raman images can be directly overlaid with no requirement for additional image correlation.
Register now to watch the full on demand webinar
Spectroscopy eBook: The Latest Advances in Correlative Raman Imaging
Raman spectroscopy is a rapidly expanding field, with modern Raman spectrometers offering labs higher ease-of-use and sensitivity. Learn how you can combine Raman spectroscopy with scanning electron microscopy (SEM) or fluorescence-lifetime imaging microscopy (FLIM), thus enhancing the technique for various applications.
- Developments in Raman instrumentation and the innovations of Raman technology;
- In-situ Raman spectroscopy within a SEM chamber, and how the inLux SEM Raman interface provides complementary information during SEM imaging;
- Correlative FLIM and Raman microscopy, with examples of imaging on plant tissue sections and HeLa cells.
Spectroscopy eBook download


