Classifying Brain Tumours with Raman Spectroscopy
How can a surgeon accurately distinguish tumour tissue from healthy tissue in under 15 minutes? The answer could be to use Raman spectroscopy.
Surgical removal of a tumour is an important treatment strategy for cancer patients, which can drastically improve their prognosis. On the other hand, removal of surrounding non-cancerous tissue can cause a great deal of additional damage to patient health. Thus, the desire to remove as much tumour tissue as possible, combined with a necessity to preserve healthy tissue, makes accurate separation of the two incredibly important.
Nowhere is this fine line between improving patient prognosis and causing additional damage more evident than in surgical neuro-oncology, where removal of functional brain tissue can cause permanent disability. To complicate matters further, a definitive boundary between tumour and healthy tissue rarely exists as cancer cells can break away from the main tumour and infiltrate surrounding non-cancerous tissue. Surgeons are therefore left with a difficult decision regarding the amount of tumour they risk removing, and a strategy to accurately differentiate between tumour and healthy tissue is highly sought after. Raman spectroscopy has the potential to offer a novel and effective approach to achieve this.
Raman Spectroscopy in the Clinic
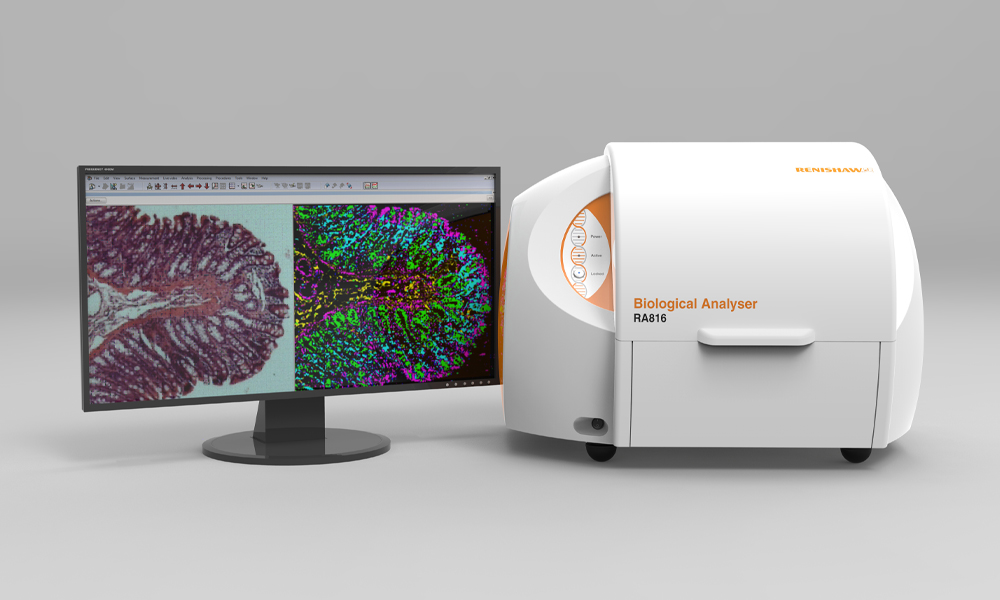
Renishaw has developed a Raman system, the RA816, which is specifically designed to facilitate biological sample analysis. Importantly, the system is compact and can be placed on a benchtop, which has allowed it to be incorporated within a clinical setting. James Livermore, a neurosurgeon within Professor Plaha's group at the Nuffield Department of Clinical Neurosciences, University of Oxford, has worked with Renishaw to develop a workflow which allows excised surgical tissue to be analysed rapidly and with minimal sample preparation. This enables Raman spectroscopic information to be obtained in under 15 minutes. With such a rapid turnaround time, Raman spectroscopy may be able to assist with real-time decision-making during operation procedures.
Tumour or Healthy Tissue?
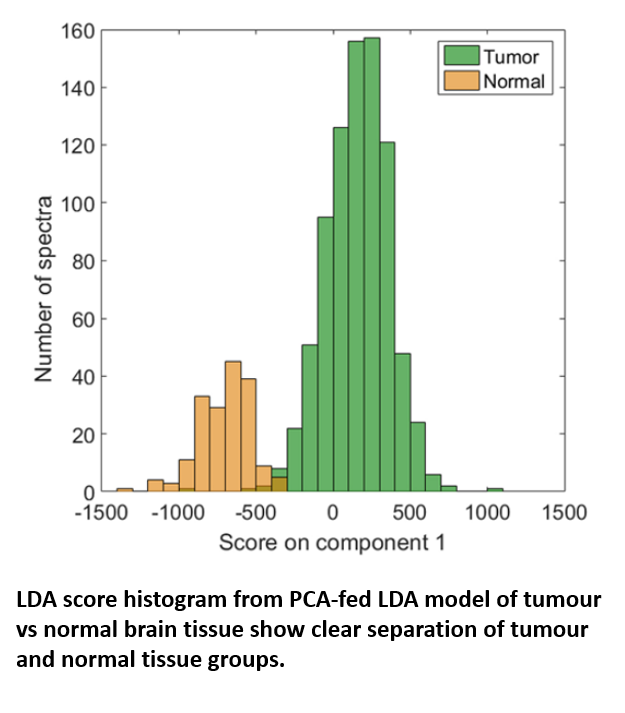
Using the RA816, James collected 9,799 Raman spectra across 62 cancerous brain biopsies and 1,825 spectra across 11 normal brain biopsies. These spectra were used to generate a classification model which could then be applied to further biopsy samples to identify them as either cancerous or non-cancerous based on small spectral differences. The model, which was built via a combination of principle component analysis (PCA) and linear discriminant analysis (LDA), achieved both sensitivity and specificity above 0.96 when identifying either tumour or normal tissue, confirming the effectiveness with which tumour and healthy tissue could be separated.
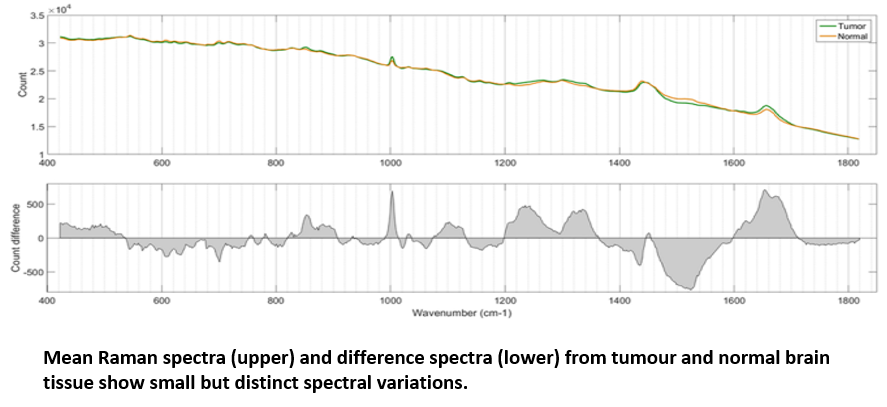
Genetic Subtyping of Tumour Tissue
Another difficulty faced by neurosurgeons arises from the fact that different patients show different responses to tumour resection. Thus, even once tumour tissue has been identified and removed, it is difficult to determine the extent and duration of the resulting improvement in patient health, as well as ascertaining the likelihood of the tumour regrowing. The potential health benefits of tumour removal are influenced by the underlying genetics of the tumour, for example, mutations of the enzyme, isocitrate dehydrogenase (IDH) are suggestive of better progression-free survival. Thus, classification of tumour tissue into specific genetic subtypes can provide valuable information for surgeons when deciding tumour resection strategies.
Raman spectroscopy has shown promise that it could offer an effective platform for classifying gliomas. James utilised the 9,799 Raman spectra from cancerous brain biopsies to generate a genetic subtyping model, which could then be employed to categorise glioma tissue into the three most common genetic subtypes: 1) astrocytoma with IDH mutation; 2) astrocytoma without IDH mutation (wild-type); and 3) oligodendroglioma. The PCA/LCA-built model effectively classified fresh tissue into each of the subtypes, with a minimum sensitivity of 0.79 and a minimum specificity of 0.90.
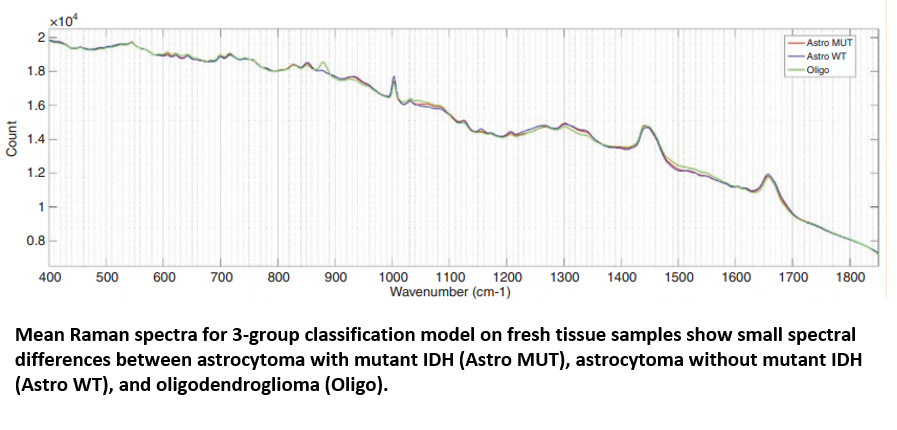

Conclusion
Thus, Raman spectroscopy can provide surgeons with a wealth of information which could support them in deciding on the most appropriate intervention strategies. It is possible that in under 15 minutes, tumour tissue could be accurately identified and marked for potential surgical resection. In addition, if there is a risk of causing damage through healthy tissue removal, the ability to rapidly acquire genetic information from the tumour could assist the surgeon in predicting patient prognosis and consequently help them to decide how aggressively to pursue resection.
Ultimately, with translation of Raman spectroscopy towards a clinical setting, healthcare professionals are provided with new options for disease diagnosis and treatment monitoring, which can be used to compliment more traditional tissue analysis approaches. Renishaw is continuing to push the boundaries of Raman-based imaging, and neuro-oncology is just one of the many application areas being explored across the life sciences.
For more detailed information, please see the following publications:
An interview with the researcher behind the work, James Livermore, can also be seen here:
Medical research using Raman Spectroscopy - YouTube
The Renishaw Biological Analyzer is designed for Research Use Only (RUO) and not for use in diagnostic procedures.
About the author
Dale Boorman, Application Scientist

Dale is an Application Scientist with six years' experience of developing and operating microscopy and spectroscopy systems for biological and medical research.
Further reading
We have a whole range or articles, case studies and news stories about Raman spectroscopy.
About the RA816 biological analyser
Discover more about how the RA816 biological analyser is suitable for your organisation's applications.